AI Event Transcription and Summaries for Pharmaceutical Roundtables
Pharmaceutical roundtables bring together medical leaders, researchers, regulators, and commercial teams and in just a few hours, they generate months’ worth of insight. Yet most of that value disappears the moment the discussion ends. Rozie Synopsis, an award-winning live-event intelligence platform, solves this gap with AI-powered transcription and structured summaries that surface in real time during the conversation and evolve into a searchable, compliant knowledge hub your teams can revisit long after the roundtable closes.

The Challenge of Capturing & Retaining Insights at Pharmaceutical Roundtables

Pharma discussions are dense, fast-moving, and highly technical. According to the well-known Forgetting Curve, people lose most new information within hours and this accelerates in environments where complex data, clinical nuance, and multiple perspectives collide in rapid succession.
Rozie Synopsis eliminates that loss by capturing every discussion in real time and converting it into accurate transcripts, structured insights, and action items that teams can review, share, and build on. It becomes the long-tail asset these highly strategic sessions have always needed.
Rozie Synopsis eliminates that loss by capturing every discussion in real time and converting it into accurate transcripts, structured insights, and action items that teams can review, share, and build on. It becomes the long-tail asset these highly strategic sessions have always needed.
What Is AI Transcription & Summarisation for Pharmaceutical Roundtables?
AI transcription turns spoken contributions from clinicians, MSLs, HEOR leads, compliance stakeholders, and industry experts into clean, speaker-tagged text using models tuned for technical vocabulary. AI summarisation then goes further analysing multi-stakeholder debates, differentiating viewpoints, and extracting structured takeaways that teams can understand at a glance.
For Participants
Stay aligned on recommendations, risks, scientific points of view, and next-step responsibilities.
For Medical & Scientific Teams
Accelerate the creation of compliant summaries, internal reviews, and evidence-based follow-up.
For Commercial & Market Access Teams
Turn expert insights into messaging frameworks, training content, and strategic planning inputs.
Trusted by global events:
















































Why Rozie Synopsis Is Valuable for Pharmaceutical Roundtables
Knowledge Retention
Revisit complex scientific discussions, expert perspectives, and decision pathways at any time.
Terminology Accuracy
Industry-specific language models recognise clinical, regulatory, and therapeutic-area vocabulary with higher precision.
Organisational Learning
AI identifies themes, recurring barriers, shared concerns, and future-of-care trends that inform strategic planning.
Content Creation
Convert Pharmaceutical roundtable conversations into medical affairs summaries, training materials, internal memos, advisory reports, and newsletters with dramatically less manual effort.
How Rozie Synopsis Works During Pharmaceutical Roundtables
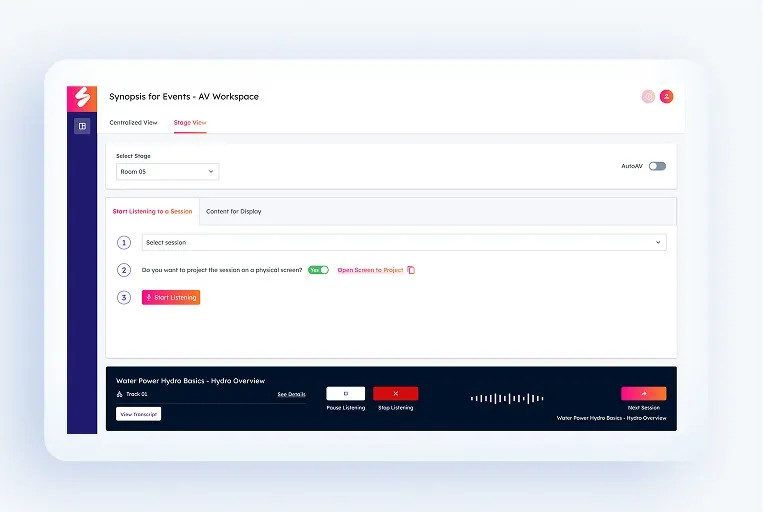
Capture Every Session
Integrates with event AV or virtual feeds to capture audio automatically.

Summarise and Analyse
AI surfaces key themes, scientific consensus, and recommended actions for medical, commercial, and compliance teams.

Transcribe Instantly
AI produces clean, speaker-tagged transcripts in real time, supported by human editorial review in high-stakes environments.
Reuse & Share
All content lives in a secure, searchable knowledge hub tailored to your event.
Award-winning AI experience production platform for events

Event Technology Awards 2025 Winner
best use of ai technology
.webp)
Event Technology Awards 2025 Winner
best technology start-up
.webp)
EVENT TECH LIVE STARTUP LAUNCHPAD WINNER 2025
gold price

Types of Pharmaceutical Roundtables Rozie Synopsis Supports
Medical Affairs & Scientific Roundtables
Capture clinician perspectives, therapeutic insights, unmet needs, and scientific debates along with the next steps for medical strategy.
Regulatory & Compliance Discussions
Preserve context around policy interpretation, guidance updates, and cross-functional decision making, with strict role-based permissions.
Market Access & HEOR Sessions
Document payer insights, evidence gaps, pricing and access debates, and strategic actions for follow-up.
R&D, Innovation & Pipeline Roundtables
Record discussions around emerging science, target selection, clinical trial design considerations, and future research priorities.
After the Roundtable: Your Post-Event Knowledge Hub

Rozie Synopsis converts every transcript, summary, and expert takeaway into a structured post-event knowledge hub fully customised to your organisation’s branding.
Instead of scattered notes, lost action items, and inconsistent recall, you get an organised, multilingual library that supports medical affairs, regulatory alignment, internal comms, training, and long-term planning.
Instead of scattered notes, lost action items, and inconsistent recall, you get an organised, multilingual library that supports medical affairs, regulatory alignment, internal comms, training, and long-term planning.
Frequently asked questions
Can Rozie Synopsis manage pharmaceutical roundtables with multiple simultaneous sessions?
Yes. Rozie supports multi-track events and captures audio across numerous rooms at once each with its own transcript and summary.
How quickly are transcripts and summaries available?
Rozie delivers real-time transcripts and near-instant summaries, allowing teams to circulate compliant recaps within minutes.
Can Rozie support global, multilingual pharmaceutical audiences?
Absolutely. Real-time translation across 20+ languages ensures speakers and participants can follow along in their preferred language.
How does Rozie help teams turn roundtable content into reusable assets?
Rozie transforms transcripts and summaries into structured insights your teams can repurpose into medical summaries, evidence briefs, internal reports, and training content.
Is Rozie suitable for sensitive or confidential pharmaceutical discussions?
Yes. Enterprise-grade security, role-based permissions, and gated hubs ensure full privacy and compliance for high-stakes conversations.
Love the product and so pleased with the output, now powered by Gemini! Getting near real time summaries of sessions from our events in user friendly interface that integrates with our event app Whova. Look forward to our continued partnership for future events
Virginia Sharma
Head of Brand and Product Marketing, Google Public Sector

I have to say that about 20 minutes into the opening presentation, that I realized that the “Speaker Insights” projected on the screen were AI generated. I will go back and refer to some of them. Well done!”
Pascale Batchoun
Speaker, IATA World Data Symposium

Rozie Synopsis tool for real time extraction of Speaker Insights is really amazing! Totally recommend this for any future conference. Great job.
Santiago Erroz Ferrer
Speaker, IATA World Data Symposium

It was really easy… and very valuable.The Synopsis team was very fast
Jakeline Anguiano
Encore (AV Tech)

Having the summary allows me to participate in a way where I get the knowledge but don’t have to choose between going here or going there.
Franklin
Attendee, ITC Vegas


